Attempts to understand the neural network inside the brain has been an area of interest for a long time and now there is a renewed interest in trying to communicate with the brain. The concept of Brain-Machine Interface (BMIs) dates back to the middle of the 20th-century [1]. Although there were attempts to probe into the brains of Monkeys early on, the attempts at probing the human brain could be traced back towards the late 20th century.
Thanks to the massive improvements in technology, scientists are now exploring possibilities of wireless implantable devices that can communicate with the human brain directly. Brain-machine interfaces(BMIs) could help in the restoration of sensory and motor function in patients of neurological disorders. The work of Elon Musk and Neuralink [2] is unparalleled in this regard and we will be discussing their work, risks, and challenges.
In the study of neural signals there are mainly two approaches, the Invasive and the Non-Invasive. The Non-Invasive approaches like Electroencephalography (EEG) suffer from poor temporal and spatial resolutions. The brain signals are not strong enough to be accurately determined by such Non-Invasive approaches. So, technically you have to arrive at Invasive approaches for better accuracy.
The minimally invasive technologies like Electrocorticography (ECoG) although require surgery but are still limited to the surface of the brain and don’t go into the brain. The Neuralink project attempts to communicate at the neuron level and unlike a one-way signal measurement in EEG and ECoG, the paper claims to also have achieved to electrically stimulate the neurons.
The Neuralink BMI
The white paper by Elon Musk and Neuralink [2] mainly discusses the following with respect to their BMI. The neural probes for interfacing, the robot to insert the probes. the electronics involved in the BMI system and the analysis of the signals captured by the system
The bio-compatible probes are based on polyimide, a proven material for such bio-electronic applications. Each probe has 32 electrodes and a collection of probes(~48 or 96), called the ‘arrays’ connect to a custom ASIC.
The robot design is very robust with its needle pincher cartridge (NPC), the Low-force contact brain position sensor, Light modules with multiple independent wavelengths, four cameras for focus during needle insertion, and Stereoscopic cameras. The robot is a self-sufficient marvel of its own and interested readers could delve into it.
The data flowing from such a large number of electrode channels would have to be compressed at the source. The electronics involving the custom ASIC and onboard FPGA handle this task and pass the processed data through USB connector for full bandwidth transfer. The PCB also involves real-time temperature, accelerometer, and magnetometer sensors, which could be of use even independent of the neural probes.
Unlike in the conventional neurophysiology where you store the neural signals and analyze it offline, Neuralink takes an on-the-fly approach by real-time detection of the spikes (Action Potential) with an online detection algorithm. The author’s claim that through a permissive filter with an estimated false positive rate of ∼0.2 Hz the conventional time-consuming approach to detect false positives (data not shown in the paper) can be avoided.
The Neural Probes
The authors claim to have designed the ASIC to be capable of electrical stimulation on every channel but no data is provided for the same. Although it is technically possible of electrical stimulation by the ASIC, but the way the stimulation could reach the neurons through the neural probes is technically challenging. This is of particular concern in cases where the sensing area and the stimulation area could be the same.
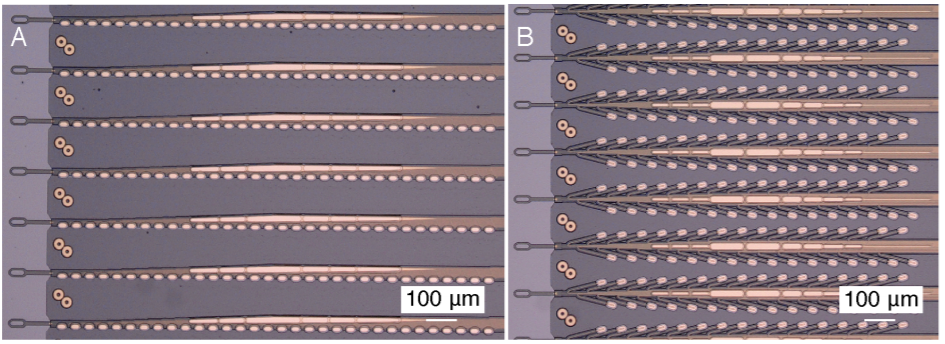
probes with 32 electrode contacts spaced by 75 µm. (source: Neuralink[2])
There may not be a race condition between the electrons and the neurons if the neural probes are being used for stimulation too. However, the loss of sensory input during stimulation and the stimulating signal artefacts can’t be ignored. The work of Andy Zhou et al [3] towards artefact-free stimulation with the use of advanced techniques (Dynamic range increasing and memory sampling) can help address some of these issues. But it is to be noted that the sensing and stimulating areas are different in the cited study. An alternate approach would be to use optogenetics [4] for stimulation on a parallel channel.
We can’t ignore the threat of the possible overlaps between a loosely bound bunch of neural probes assuming the tightly bound probes would result in strain on the probes and an eventual breakdown. The wear and tear of the probes over the years may cause them to become obsolete. The use of bio-degradables in traditional medical devices is for the reason that after the term of use we need not have to perform another surgery to take the device out. But, for the probes designed to reside in the body virtually forever, the potential use of bio-degradable materials is also questionable.
Wireless
The BMI has a wired USB connection for both power and communication. To make it truly an implantable device, the device has to be wireless. Given the high bandwidth data that the BMI has to drive and the low power budget for an implantable device, existing wireless technologies are not feasible.
According to a study on minimum requirements for real-time spike detection [5], it would require sampling at 25kHz which results in roughly a data of 500 Kbit/s per channel. Even if we consider the sampling rate of ADCs given in the Neuralink paper, 19.3 kHz with 10-bit resolution, the throughput is easily of the orders of Mbps for a sum total of traffic from all the channels. The default choice for most medical devices is the Bluetooth and its low power version, the BLE has a practical throughput of ~100 kbps [6].
The situation demands novel approaches towards wireless data compression, radio design, and the way we look at the neural signals. The work of Andy Zhou et al [3] with the use of local field potentials (LFPs) is significant in this regard. The local field potential (LFP) is a summation signal of excitatory (EPSP) and inhibitory (IPSP) dendritic potentials from a large number of neurons in the neighborhood of the probing site. Compared to the neuron level Action Potential(AP), the LFP would reduce the wireless bandwidth for real-time data transfer. Needless to say, this approach would compromise the resolution.
Power
The Neuralink ASIC requires 6mW of power and the system would need 550mW for 1,536 channels. Each ASIC can handle 256 channels, so for the 1,536 channels we would need 6 such ASICs which could roughly make 36mW of 550mW. The rest power consumption would be shared by the on-board FPGA and the data transfer via USB. Figure below shows the assembly of ASICs in the system.
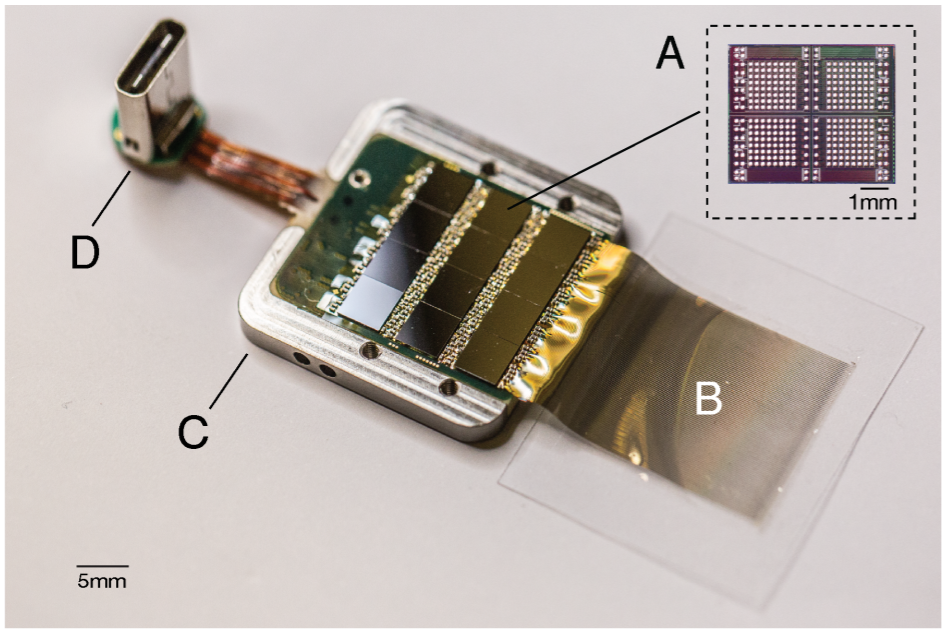
The results demonstrated in the paper are based on experiments on the Long-Evans rats and for a human brain we may need more channels, thereby pushing more mW to the power budget. The power management techniques from the SoC design could be used to avoid excessive power drain. Instead of having an always-on approach for all the ASICS, they could be switchable. This would need a careful understanding of their inter-relations between brain regions and the associated neural probes.
To Explore the options for the power mechanism for such a wireless system we have to consider that we need both high power density and high energy density. The approaches considered for medical applications including energy harnessing from the body provide negligible power(of orders of nW). This makes it for the case of the most common choice of Li-ion or Li-polymer batteries.
Security
If we consider that a wireless implantable Brain-Machine Interface can be implemented overcoming all the challenges, one of the most important and perpetual challenge would be its security. Security for a device like this should be of utmost priority just like the functionality of the device. We could dedicate a whole blog on the security challenges faced by computer systems in general and Bio-medical devices in particular. Interested readers can go through the review article on security issues of implantable devices [7]. One possible scenario of an advanced Side-channel attack for such a device could be the unintended leakage of the thoughts or emotions of a person based on which of the ASICs are active for most of the time (assuming we have switchable ASICs, as discussed earlier) or based on the power dissipation pattern of the device.
Conclusion
One of the major advantages of the design is its modular approach with respect to the ASICs. This improves design re-usability for addressing different needs. The robust robot design is very advantageous with respect to the installation of the system, as it supports an auto-insertion mode which can insert up to 6 probes per minute. Although details are not shared on the comparison of online spike detection algorithm against the conventional offline spike sorting algorithms, the citations [8] give a hint towards such possibilities. The aforementioned challenges with respect to Probes, Connectivity, Power, and Security appear to be significant blockades in the way ahead of a wireless implantable high-bandwidth closed-loop BMI.
The scope of such a device that is said to be available within a year [9] could be assumed to be limited by electrodes. Few electrodes with only a couple of ASICs, and hence reduced bandwidth and power requirements is manageable by existing technologies. This could serve the purpose of sensing and a stimulating a specific activity. However, the idea of machines taking over the brain in a transhumanistic understanding is limited at the engineering end as stated above.
In the book ‘The Attention Merchants’, the author illustrates how the access of technology to our bed rooms has captured human attention. Technologies like these are projecting direct access to our brains and such a future is both intriguing and intimidating. However, technologies like these, could help us better understand the brain and thereby treat neurological disorders like Epilepsy, Parkinson’s, and Alzheimer’s, etc. Therefore, we could look forward to such technologies in this regard.
References
[1] E. V. Evarts, “Pyramidal tract activity associated with a conditioned hand movement in the monkey.,” Journal of Neurophysiology, vol. 29, no. 6, pp. 1011-1027, 1966.
[2] E. Musk et al., “An integrated brain-machine interface platform with thousands of channels,” Journal of medical Internet research, vol. 21, no. 10, p. e16194, 2019.
[3] A. Zhou, S. R. Santacruz, B. C. Johnson, G. Alexandrov, A. Moin, F. L. Burghardt, J. M. Rabaey, J. M. Carmena, and R. Muller, “A wireless and artefact-free 128-channel neuromodulation device for closed-loop stimulation and recording in non-human primates,” Nature biomedical engineering, vol. 3, no. 1, pp. 15-26, 2019.
[4] M. Bolus, A. Willats, C. Whitmire, C. Rozell, and G. Stanley, “Design strategies for dynamic closed-loop optogenetic neurocontrol in vivo,” Journal of neural engineering, vol. 15, no. 2, p. 026011, 2018.
[5] J. Navajas, D. Y. Barsakcioglu, A. Eftekhar, A. Jackson, T. G. Constandinou, and R. Q. Quiroga, “Minimum requirements for accurate and efficient real-time on-chip spike sorting,” Journal of neuroscience methods, vol. 230, pp. 51-64, 2014.
[6] J. Tosi, F. Taffoni, M. Santacatterina, R. Sannino, and D. Formica, “Performance evaluation of Bluetooth low energy: a systematic review,” Sensors, vol. 17, no. 12, p. 2898, 2017.
[7] C. Camara, P. Peris-Lopez, and J. E. Tapiador, “Security and privacy issues in implantable medical devices: A comprehensive survey,” Journal of biomedical informatics, vol. 55, pp. 272-289, 2015.
[8] E. M. Trautmann, S. D. Stavisky, S. Lahiri, K. C. Ames, M. T. Kaufman, D. J. O’Shea, S. Vyas, X. Sun, S. I. Ryu, S. Ganguli, and K. V. Shenoy, “Accurate estimation of neural population dynamics without spike sorting,” Neuron, vol. 103, no. 2, pp. 292 – 308.e4, 2019. [9] https://www.businessinsider.com/elon-musk-neuralink-brain-chip-put-in-human-within-year-2020-5